Explain Why H2o and Co2 Molecule Have Different Shape
Similar H2o Consists Of Polar Molecules Co2 Consists Of Nonpolar Molecules How Do Chemists Explain This Difference Quora


Molecular Structure Of Co2 Carbon Dioxide Youtube
Is Co2 Linear While So2 Is Bent Quora

Assertion And Reason Both Are Correct Statements And Reason Is Correct Explanation For Assertion
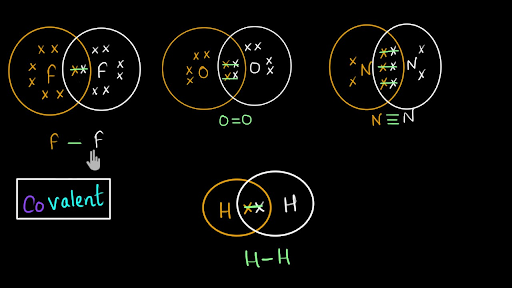
Covalent Bond And Lewis Dot Structure H2o Co2 Video Khan Academy

Although Both Co2 And H2o Are Triatomic Molecules The Shape Of H2o Molecule Is Bent While

Do Carbon Dioxide And Water Have The Same Molecular Geometry If Not What Attributes Cause These Brainly Com

Co2 Lewis Structure Molecular Geometry Molar Mass Hybridization

Co2 Lewis Structure Molecular Geometry Molar Mass Hybridization

Quantum Mechanics Bond Angles H2o Vs Co2 Physics Stack Exchange
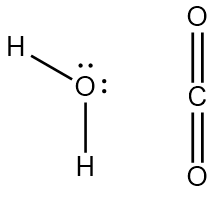
Which Statement Best Explains Why The Polarity Of A H2o Molecule Differs From The Polarity Of A Co2 Molecule A The Bond Strength Is Greater In Co2 B The Central Atom In

Although Both Co2 And H2o Are Triatomic Molecules The Shape Of H2o Molecule Is Bent While That Of Co2 Is Linear Explain This On The Basis Of Dipole Moment

Co2 Lewis Structure Molecular Geometry Molar Mass Hybridization
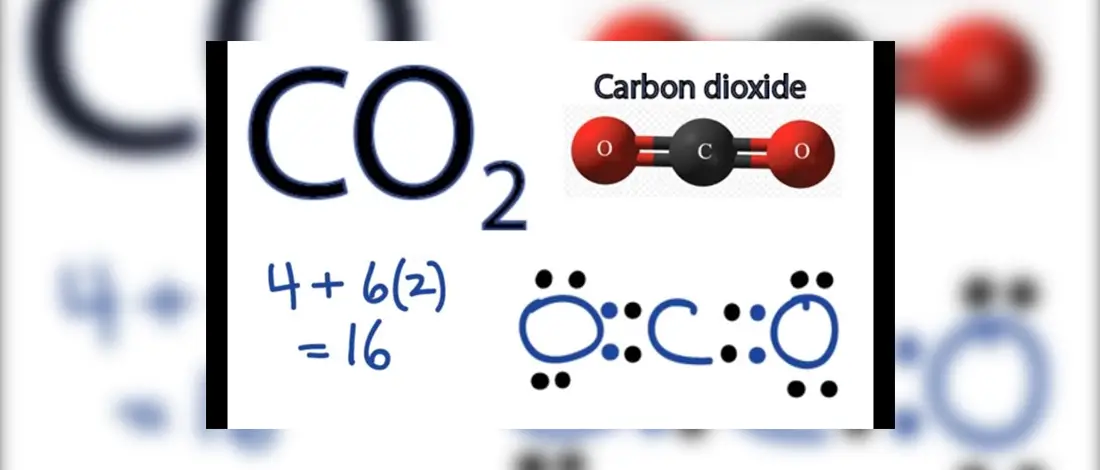
Co2 Lewis Structure 2021 Updated All You Need To Know

Explain Why H2o Has Dipole Moment While Co2 Does Not Have Brainly In

Is Co2 Polar Or Nonpolar Techiescientist

Give Reasons I Water Molecule Has Bent Structure Whereas Carbon Dioxide Molecule Is Linear Ii All The C O Bonds In Carbonate Ion Are Equal In Length
Carbon Dioxide And Water Molecules Both Have Three Atoms However Carbon Dioxide Is Linear While Water Is Bent How Do You Account For The Difference In Their Molecular Geometries Quora

Comments
Post a Comment